LABORATORY ANALYSIS OVERVIEW
Spartan contains GW-501516 at a labeled dosage of 25 mg per capsule, intended for laboratory research purposes only.
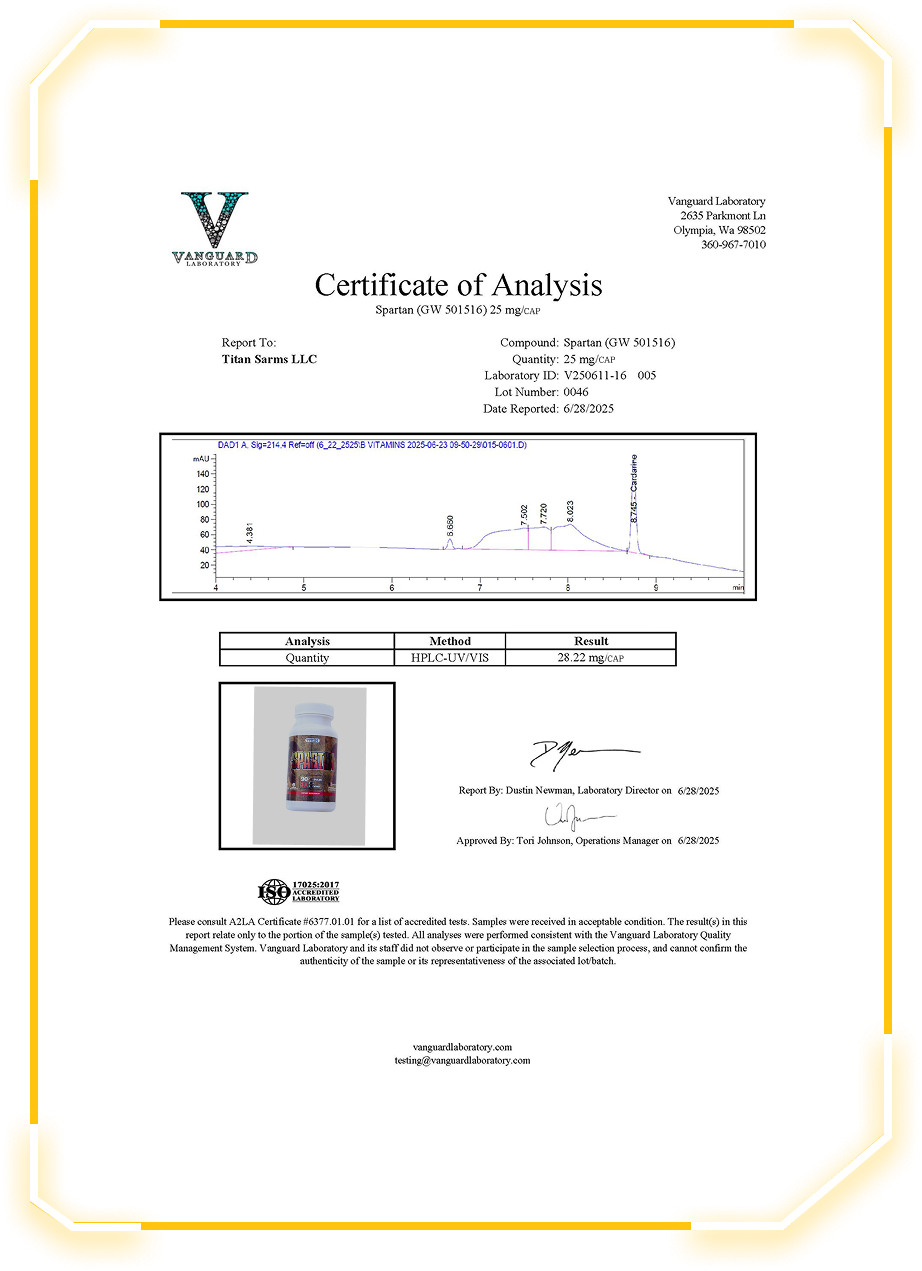
Quantitative testing found 28.22 mg/CAP, indicating a slightly overfilled capsule relative to the label. This suggests consistent or possibly conservative formulation practices for research-grade reliability.
High-Performance Liquid Chromatography with UV/Visible detection (HPLC-UV/VIS) was used to quantify GW-501516 content, which is standard for verifying compound purity and concentration.
The report includes a chromatogram trace showing peak retention time at approximately 8.74 min, aligning with known standards for Cardarine. Minimal signal noise or unexpected peaks were detected, supporting compound integrity.
Lot Number: 0046
Laboratory ID: V250611-16 005
Date Reported: 06/28/2025
These identifiers ensure the compound is traceable back to its testing instance and batch, which is essential for regulated research environments.
Testing was conducted by Vanguard Laboratory, an ISO/IEC 17025:2017 accredited facility. While they confirm results on the tested sample, they do not validate the full batch, and results should be interpreted accordingly in controlled settings.